Abstract
Background: CT-P10 is an approved biosimilar to the innovator rituximab (RTX) from many countries including European Union based in part on the pharmacokinetics (PK) equivalence and comparable efficacy in patients with previously untreated advanced follicular lymphoma (FL) when treated with rituximab plus cyclophosphamide, vincristine and prednisone (R-CVP) as an induction therapy (Coiffier B et al. ASH 2016; Kim WS et al. ASCO 2017).
Objective: We report here the updated efficacy outcomes including progression free survival (PFS), duration of response, overall survival (OS), as well as updated safety profile of CT-P10 compared to RTX in advanced FL patients with median follow-up duration of 23 months including the Maintenance Period with rituximab monotherapy.
Methods: These results were derived from an ongoing randomized and double-blind study in patients with previously untreated advanced FL (NCT02162771). A total of 140 patients were randomized in a 1:1 ratio and 124 patients completed 8 cycles of R-CVP induction therapy. One-hundred twenty two patients (62 patients in CT-P10 group and 60 patients in RTX group), who showed response during the Induction Period, entered the Maintenance Period where a total of 12 cycles of rituximab monotherapy was to be administered every 2 months. The study was planned to continue until death or up to 3 years from the randomized date of the last patient. Kaplan Meier (KM) method was used to estimate PFS, duration of response, and OS.
Results: Both groups had similar baseline characteristics; overall median age of 58 years, 55% female, 57% with FLIPI score ≥3, 100% with Stage III/IV, 18% with bulky disease (≥7cm) and 26% with B-Symptom.
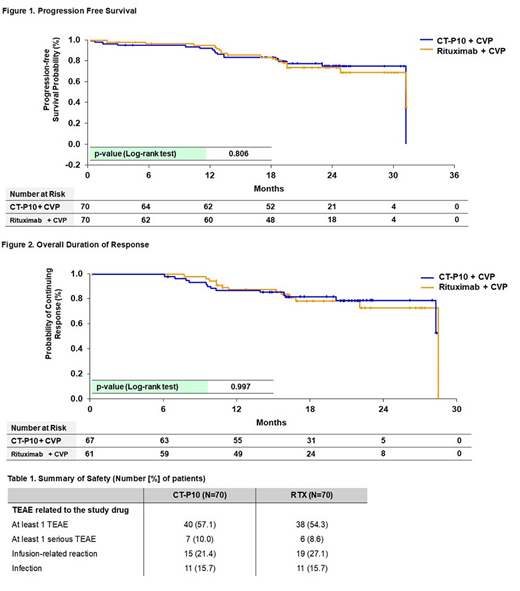
As of the cut-off date for investigator-assessed PFS, duration of response and OS, median follow-up was 23 months (range, 0.5-34) in the CT-P10 group and 22 months (range, 0.2-33) in the RTX group. The proportion of patients who had experienced relapse, disease progression or death from any cause was 22.9% (16/70) and 24.3% (17/70) for the CT-P10 and RTX groups, respectively. There was no significant difference between CT-P10 and RTX groups in PFS (log rank, p-value: 0.806) with 2-year PFS of 75.2% and 73.5%, respectively (Figure 1). In terms of sustained response, the proportions of patient who showed relapse or disease progression after achieving overall response (Complete Response, unconfirmed Complete Response, or Partial Response) were 19.4% (13/67) in CT-P10 group and 21.3% (13/61) in RTX group, and the KM curves showed no statistically significant difference between CT-P10 and RTX (log rank, p-value: 0.997) (Figure 2). Death from any cause were 5.7% (4/70) and 2.9% (2/70) in the CT-P10 and RTX groups, respectively. There was no statistically significant difference in OS (log rank, p-value: 0.464) between the CT-P10 and RTX groups with 2-year OS of 93.2% and 95.3%, respectively.
Overall safety profile of CT-P10 was consistent with that of RTX (Table 1). A similar number of patients in each treatment group experienced at least 1 Treatment Emergent Adverse Events (TEAE) considered to be related to the study drug, infusion-related reaction, and infection. The proportion of patients with positive anti-drug antibody was also similar in both groups (4.3% [3/70] vs 5.7% [4/70] in the CT-P10 and RTX groups). Neither progressive multifocal leukoencephalopathy nor Hepatitis B virus reactivation was reported in either group.
Conclusion: At the median follow-up duration of 23 months, the updated efficacy data in advanced FL patients demonstrated comparable PFS, sustained response and OS between CT-P10 and RTX. CT-P10 was also well tolerated and its safety profile was similar to that of RTX. The updated safety results did not reveal any trends or new signals noted in the patients treated with CT-P10.
Kim:Mundipharma: Research Funding; Novartis: Research Funding; Kyowa-Kirin: Research Funding; Celltrion: Research Funding; Roche: Research Funding; J&J: Research Funding; Takeda: Research Funding. Buske:Roche: Honoraria, Research Funding; Bayer: Research Funding; Janssen: Honoraria, Research Funding. Ogura:MeijiSeika Pharma: Consultancy; Celltrion: Consultancy, Research Funding; Mundi Pharma: Consultancy; SymBio: Research Funding; Takeda: Honoraria; Cellgene: Honoraria. Coiffier:CELGENE: Consultancy, Membership on an entity's Board of Directors or advisory committees; MUNDIPHARMA: Membership on an entity's Board of Directors or advisory committees; CELLTRION: Membership on an entity's Board of Directors or advisory committees; MORPHOSYS: Membership on an entity's Board of Directors or advisory committees; NOVARTIS: Membership on an entity's Board of Directors or advisory committees. Lee:Celltrion, Inc: Employment. Kim:Celltrion, Inc: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal